Background: Chimeric antigen receptor (CAR)-T cells have achieved great success in treating hematological malignancies. However, the management process during hospitalization can be complicated by the adverse events of coagulopathy. CAR-T-associated coagulopathy (CARAC) has a high prevalence and may develop into disseminated intravascular coagulation in severe cases. Meanwhile, the underlying mechanisms have not been fully elucidated. Our study aims to comprehensively analyze the profiles of CARAC and explore its potential pathogenesis.
Methods: We collected peripheral blood samples from patients received CAR-T therapy in two cohorts at Wuhan Union Hospital. Cohort 1 included patients with acute B-lymphoblastic leukemia (B-ALL) from August 2019 to March 2021, and cohort 2 included patients with ALL and lymphoma from December 2021 to December 2022. Samples were collected at various timepoints, including before fludarabine/cyclophosphamide lymphodepletion (pre-FC), D0, D7, D14, D28, and at the peak of IL-6 after CAR-T cell transfusion. In cohort 1, we analyzed the peripheral blood protein profiles using proteomics, and in cohort 2, we verified the fibrinolysis-related indicators. The diagnosis of CARAC was based on the consensus of CARAC in 2022, and the CRS classification followed the ASTCT standard.
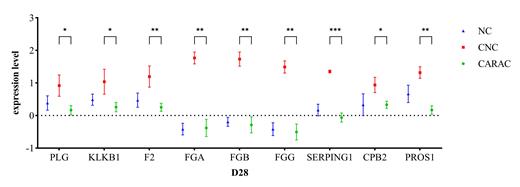
Results: We found no significant differences in baseline characteristics among the patients in cohort 1 (n=20). All CARAC patients (n=10) had CRS, with half of them (n=5) having G≥3 CRS. In contrast, patients with CRS but not CARAC (CNC, n=3) had only G1-2 CRS, indicating that severe CRS is more likely to be associated with CARAC, consistent with our previous study. We identified 293 differentially expressed proteins (DEPs, p<0.05, |log2FC|>0.585) that were mostly downregulated in CARAC patients compared to CNC patients at the six timepoints. Enrichment analysis indicated that these DEPs were mainly involved in immune response, complement and coagulation pathways, fibrinolytic pathways, and acute inflammation response. We selected the top 10 DEPs with the highest degree of inter-protein correlation, including C3, C4A, C4B, MBL2, C8A, C8B, CRP, A2M, CLU, and FGA, which further suggest that complement and acute inflammation response play crucial roles in the pathogenesis of CARAC. Based on the clinical manifestations of hemorrhage in CARAC patients, hyperfibrinolysis is presumed to be the main mechanism. Interestingly, compared to patients without CRS (NC, n=7), CNC patients showed increased levels of SERPING1, CPB2, F2, FGA, FGB, FGG, KLKB1, PLG, and PROS1, which are proteins involved in the fibrinolysis pathway (p<0.05) (figure 1). Although there were no statistically significant differences, the levels of these proteins were higher in patients with G≥3 CRS compared to G1-2 CRS patients. Furthermore, compared to CNC patients, CARAC patients showed decreased levels of these proteins at D28 (p<0.05) (figure 1). Additionally, the nadirs of PLT and fibrinogen levels were markedly lower in CARAC patients compared to CNC patients, while the fibrinolytic indicators D-dimer and FDP were significantly higher in CARAC patients. Furthermore, we observed the activation of the fibrinolysis system in cohort 2. Among patients with CARAC (n=7), the levels of tPA, PAI-1, and uPAR were higher compared to patients with CNC (n=6). These findings indicate the activation of the coagulation system and hyperactivity of the fibrinolytic system in CARAC patients.
Conclusions: Through proteomic analysis of cohort 1 and validation of cohort 2, we have preliminarily revealed that the core pathogenesis of CARAC involves the network regulation of immune, inflammatory, coagulation, and fibrinolytic systems.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal